News & Trends - Pharmaceuticals
Is the nation building on the foundations of CAR-T therapy?
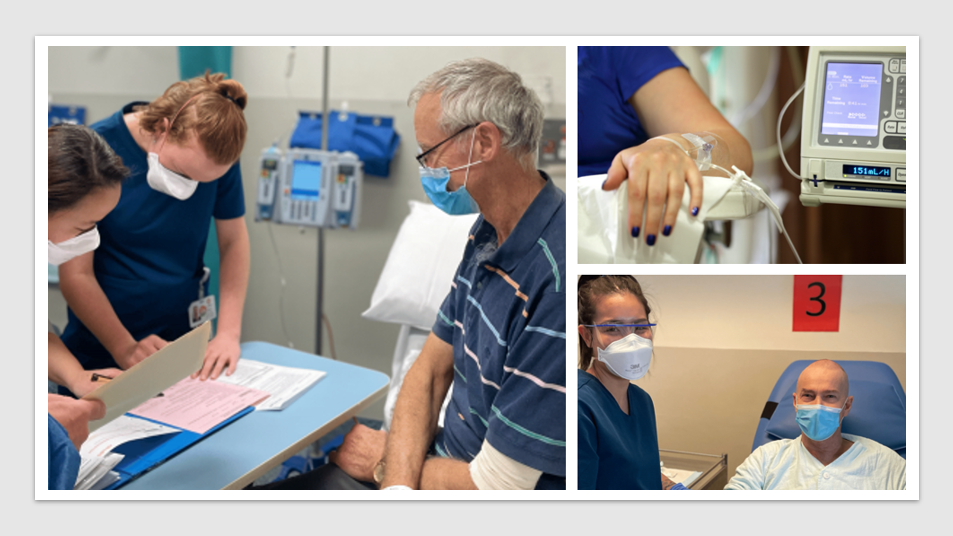
Pharma News: A state-of-the-art treatment unit designed explicitly for patients receiving cutting-edge cell-based therapies, including CAR-T cell therapy, has opened in the Peter MacCallum Cancer Centre.
Nestled on Level 1 of Peter Mac’s primary facility in Parkville, the newly established Ambulatory Cell Therapies Unit (1E) has been meticulously outfitted to cater to all facets involved in administering and overseeing patients undergoing these transformative therapies. Part of the Clinical Haematology Department, this pioneering unit commenced operations last week, initially boasting six treatment chairs complemented by dedicated clinic amenities.
The cost of current therapies stands at over AU$500,000 per patient, without government support. Presently, two CAR‐T cell therapies, Novartis’ Kymriah and Gilead’s Yescarta, catering to B‐cell cancers, are funded in Australian public hospitals through a joint federal and state government funding model. Service providers receive prospective payments based on yearly submissions of actual costs to the Independent Hospital Pricing Authority (IHPA), which has been pivotal in granting Australians early access to these therapies and gathering data for enhancements.
However, due to the intricate nature of these services and the recent commencement of data collection in 2020, a comprehensive understanding of costs and parameters is still in progress. For example, IHPA noted advice from jurisdictions “that the Relative Value Units for pathology, and used for allocating costs associated with bed days do not adequately account for the intensive nature of CAR‐T treatment”. Moving from retrospective reconciled funding to a more concurrent activity-based funding model will require time, increased patient numbers, and robust data collection to achieve.
The Medical Services Advisory Committee (MSAC) review of the clinical, cost effectiveness and budget impact of Novartis’ Kymriah was conducted in June this year. Another review is pending in three years as cost data from state and territory governments showed the true cost of providing the therapy was substantially higher than expected.
Undoubtedly, the advent of CAR T-cell therapies has been a game-changer in addressing specific cancer patient cohorts with significant unmet needs. Notably, therapies targeting CD19 on B cells have displayed remarkable complete response and remission rates of 77% and 80% in acute lymphoblastic leukaemia cases, marking a substantial leap in treatment efficacy.
However, the implementation of CAR T-cell therapy is intricate, fraught with technical and organisational challenges. Approximately 26% of treated patients encounter cytokine release syndrome, while 12% grapple with neurotoxicity – both reaching grade 3 or higher, necessitating intensive care. Nonetheless, clinicians are refining strategies to manage these toxicities while exploring innovative CAR T-cell designs and improved potency assays to mitigate complications.
Ian Oberin, a myeloma patient from Wodonga and among the unit’s initial beneficiaries, emphasised the convenience of centralised care, particularly for individuals traveling from regional areas.
“It’s the ease and convenience of not having to go to a number of locations,” Ian expressed, underscoring the unit’s practicality.
The establishment of this unit marks the culmination of three major cellular therapy initiatives at Peter Mac, all made feasible by an $80 million Federal Government investment, coupled with an additional $25 million from Peter Mac and the Peter MacCallum Cancer Foundation.
This joint investment facilitated not only the unit’s opening but also the establishment of a cutting-edge Centre of Excellence in Cellular Immunotherapy laboratory. Moreover, it supported ongoing clinical trial development to propel the science of cell-based treatments forward.
Furthermore, the investment facilitated the construction of expanded clean-room and cellular therapy manufacturing facilities on Level 9, operated by Cell Therapies, augmenting Peter Mac’s infrastructure in the field.
This initiative mirrors a global surge in regenerative medicine, including cell and gene therapies, witnessing nearly a 50% surge from US$13.5 billion in 2018 to US$19.9 billion in 2020, encompassing around 1200 ongoing clinical trials. Experts anticipate the global market to soar to AU$120 billion by 2035, potentially positioning Australia to garner AU$6 billion in annual revenue and generate ~6,000 jobs through exports and early access to pioneering treatments.
Presently, Australia hosts over 150 clinical trials and boasts nine approved gene and cell therapies, such as Biogen’s Spinraza, Gilead’s Tecartus and Yescarta, Novartis’ Kymriah, Zolgensma and Luxturna, signifying the country’s pivotal role in advancing innovative therapeutic solutions.
 In reimagining healthcare, Health Industry HubTM is the ONLY one-stop-hub uniting the diversity of Pharma, MedTech, Diagnostics & Biotech sectors to inspire meaningful change. The exclusive leadership and influencer podcasts and vodcasts offer unparalleled insights and add immense value to our breaking news coverage.
In reimagining healthcare, Health Industry HubTM is the ONLY one-stop-hub uniting the diversity of Pharma, MedTech, Diagnostics & Biotech sectors to inspire meaningful change. The exclusive leadership and influencer podcasts and vodcasts offer unparalleled insights and add immense value to our breaking news coverage.
 The Health Industry HubTM content is copyright protected. Access is available under individual user licenses. Please click here to subscribe and visit T&Cs here.
The Health Industry HubTM content is copyright protected. Access is available under individual user licenses. Please click here to subscribe and visit T&Cs here.
News & Trends - Pharmaceuticals

Horizon scanning report exposes critical systemic gaps demanding immediate reform
Medicines Australia has released the report from its 2024 Horizon Scanning Forum, a cornerstone of its Strategic Agreement with the […]
MoreNews & Trends - Pharmaceuticals

Is the NHS decline a warning for Australia’s healthcare system?
A decline in the performance of England’s National Health Service (NHS) offers important insights for Australia and other nations that […]
MoreNews & Trends - MedTech & Diagnostics

NSW’s first integrated care centre for prostate cancer patients sets new standard
In a NSW-first, a centre dedicated to the comprehensive treatment of prostate cancer, offering free, integrated care for both public […]
MoreNews & Trends - MedTech & Diagnostics

Medtronic and Abbott compete for leadership in next-gen leadless pacemakers
Medtronic is advancing its leadless pacemaker technology with the release of the Micra VR2 and Micra AV2, highlighting new features […]
More